ClarityDX Prostate is Revolutionizing Prostate Cancer Detection
perrin2024-01-10T22:05:14+00:00Disruption Magazine Special Issue 21
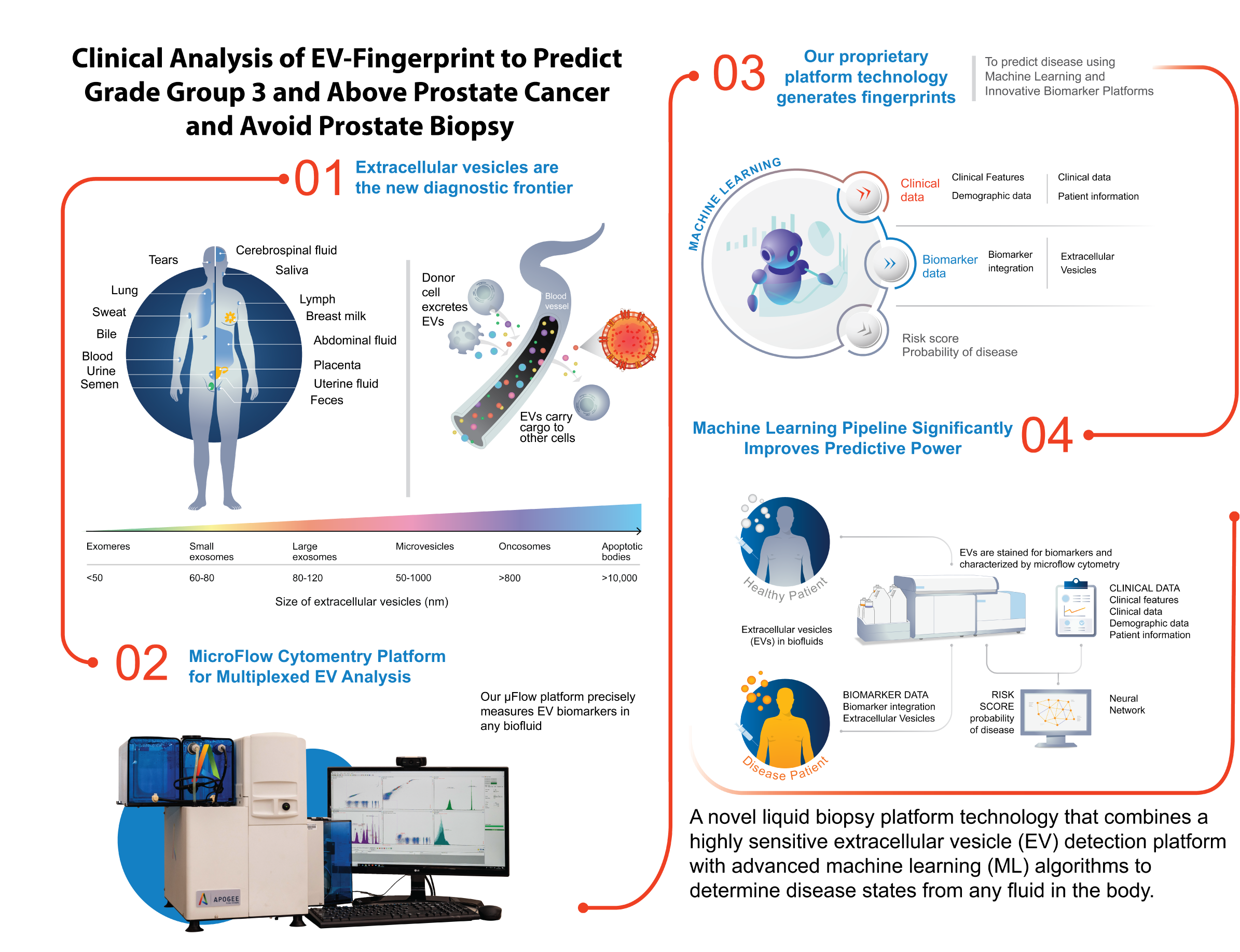
A Better Way: How Nanostics is Revolutionizing Prostate Cancer Detection
“Any jurisdiction in the world can adopt our test. It’s got huge potential.” Nanostics Chief Commercialization Officer Colin Coros
In the realm of medical innovation, success stories often find their roots in personal journeys. Such is the case with Nanostics, a pioneering company in the field of prostate cancer diagnostics. The tale begins with Frank Sojonky, an Alberta businessman, who, upon facing a prostate cancer diagnosis, embarked on a mission to revolutionize the landscape of prostate cancer research and testing.
Sojonky’s dissatisfaction with the lack of focus on prostate cancer research in Alberta led to the creation of the Bird Dogs for Prostate Cancer Research organization in partnership with the Alberta Cancer Foundation. The organization ultimately raised more than $20 million to establish a translational research program, known as the Alberta Prostate Cancer Research Initiative, headed by CEO Dr. John Lewis. It evolved into the world’s largest repository for prostate cancer patient samples, and with a focus on translation, those samples became instrumental in developing new therapeutics and diagnostic tests.
Click below to read the full article.